Continuum Mechanics is the study of the behavior of materials by ignoring its particulate nature.
A continuum is an area that can keep being divided and divided infinitely with no individual particles. It is a simplification that allows us to investigate the movement of matter on scales larger than the distances between particles.
In continuum, the
smallest element of a fluid is NOT a fluid molecule, but rather a fluid
particle that contains enough number of molecules to make meaningful
statistical averages. Continuum assumes that fluid and flow properties like pressure,
temperature, density, velocity, etc. vary continuously throughout the fluid.
This helps to study a
wide range of phenomena, from air and water flow to even the evolution of
galaxies.
For us to know whether or not the continuum hypothesis can be used, a dimensionless number called Knudsen number is used. The Knudsen number allows for characterizing the boundary conditions of a fluid flow. It is defined as:
If the length scale of
the fluidic system is in the same range as the mean free path, i.e., Kn = 1,
the fluid cannot be treated as a continuum.
The Knudsen number is very useful when assessing the boundary of fluid flows. Usually, the flow at the boundary of a flow field where the channel walls are fixed in space and the liquid directly in contact is considered to not be moving. This is referred to as the no-slip boundary condition, i.e., there is no relative movement (slip) between the wall and the fluid layer that directly contacts with the wall.
According to the Knudsen
number, flows can be divided into different regimes:
(a) For Kn < 0.01,
continuum flow dominates and conventional fluid dynamics equations are
applicable, indicating that gas molecules interact with neighboring molecules.
(b) For 0.01 < Kn < 0.1, the slip flow regime occurs when the gas molecules experience slipping at the solid interface.
(c) For 0.1 < Kn < 10, transition flow occurs. This refers to a flow regime in which both slip (continuum) and diffusion flows can occur.
(d) For Kn > 10, Knudsen's (free molecular) flow occurs. This refers to gas molecules that flow with minimal or no interaction with neighboring molecules.
Fluid Properties:
Characteristics of a
continuous fluid which are independent of the motion of the fluid are called basic
properties of the fluid. Some of the basic properties are as discussed below:
- Density: The density ρ of a fluid is its mass per unit volume (kg/m3).
- Specific Weight: This is the weight of a fluid per unit volume (N/m3).
- Specific Volume: is the volume occupied by unit mass of fluid (m3/kg).
- Specific Gravity: For liquids, it is the ratio of density of a liquid at actual conditions to the density of pure water at 101 kN/m2, and at 4°C.
- For gases, the specific gravity is the ratio of its density to that of either hydrogen or air at some specified temperature or pressure.
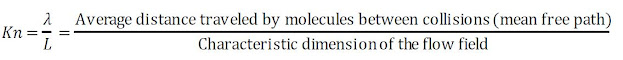













.JPG)
.JPG)

.JPG)



