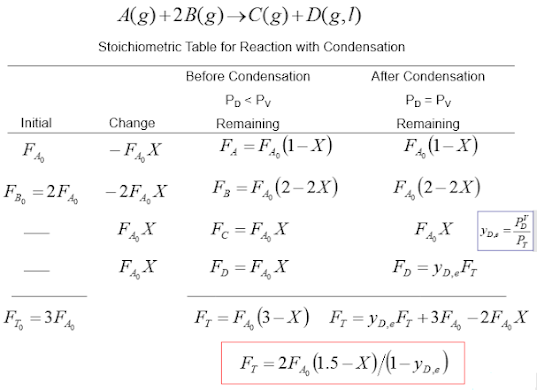
A condensation reaction is a type of a reaction where two molecules combine to form a single molecule, often water, is then removed from the reaction. For example, when two amino acid molecules combine in a condensation reaction, a covalent bond forms between the amine nitrogen of the first amino acid and the carboxyl carbon of the second amino acid. This results in a molecule of water being removed as a product. This is illustrated below:
Monday, 26 June 2023
Condensation Reactions
Monday, 12 June 2023
Short Notes #7
Catalysts
These are extremely important substances or materials used in numerous chemical processes.
Catalysts are substances that increase the reaction rate without it itself being consumed in the process. Thus, catalysts do not appear in the overall stoichiometric reaction it is used it but appears in the elementary reactions in the mechanism for the catalyzed reaction.
Examples of catalysts:
- Enzymes - These are proteins that act as catalysts in biochemical reactions.
- Homogeneous catalysts
- Heterogeneous (or surface) catalysts
Poison: A substance that reduces the efficiency of a catalyst. For example lead compounds poison platinum catalysts.
Homogenous Catalysis:
This is a process where a catalyst that is in the same phase from the reactants. Typically, everything will be present as a gas or contained in a single liquid phase.
Heterogeneous Catalysis:
This is process where a catalyst that is in a separate phase from the reactants. Typical examples involve a solid catalyst with the reactants as either liquids or gases.
Most examples of heterogeneous catalysis go through the same stages:
- One or more of the reactants are adsorbed (stick) on the surface of the catalysts on special locations called active sites (part of the surface where adsorbed materials can react with one another).
- Interaction between the catalyst surface and the reactant molecule making them for reactive through weakening of bonds in the attached molecules.
- The reaction occurs. Both the reacting molecules may attach to the surface or one might attach and the other moving freely in the liquid or gas medium.
- The product molecules desorbs from the catalyst surface i.e. breaks free from the catalyst surface to allow other reacting molecules to attach themselves and the reaction cycle starts again.
Effects of Catalysts
Catalyst lower the activation energy for a reaction. Activation energy is defined as the minimum amount of energy that is needed to a chemical reaction to occur. This is illustrated in the graph below.
Maxwell Equation Solved Example
Example Show that: Solution This example uses the Maxwell Relations Table from the previous blog entry. You can read it here Lets combine ...

-
Fluid mechanics equations helps to predict the behavior of fluids in various flow situations. A fluid can be defined as a substance that de...
-
Let us determine the pressure at any point in a fluid a rest. For this, let us consider a wedge-shaped particle that is exposed on all sides...
-
1. Vapour Power Cycles Power cycle is a process where devices are used to continuously produce power. A typical vapor power cycle is the st...