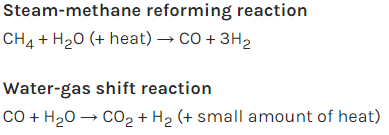Wednesday, 20 September 2023
Wednesday, 13 September 2023
Unlocking the Wonders of Silica Aerogel: Features, Applications, and Future Horizons
Silica aerogel, often referred to as "frozen smoke" or "blue smoke," is a captivating material that has captured the imagination of scientists and engineers alike. This ultra-lightweight substance boasts remarkable features, making it a standout in the world of chemical engineering. In this blog post, we will explore the most important aspects of silica aerogel, from its exceptional properties to its wide-ranging applications and future prospects.
Features of Silica Aerogel
Exceptional Porosity
Silica aerogel holds the distinction of being one of the lightest and most porous materials known to humanity. Its structure is comprised of tiny interconnected silica nanoparticles, resulting in an incredibly high surface area. Imagine a sponge, but one so porous that it appears almost weightless. This unique feature makes silica aerogel a champion in applications requiring large surface areas and minimal density.
Superb Thermal Insulation
When it comes to thermal insulation, silica aerogel reigns supreme. Its low thermal conductivity, combined with its lightness, makes it an ideal choice for extreme temperature environments. In fact, NASA has utilized silica aerogel in space missions to insulate spacecraft and protect instruments from the harsh conditions of outer space. This material's insulating prowess extends to terrestrial applications as well, such as improving the energy efficiency of buildings.
Exceptional Transparency
Silica aerogel's transparency is another captivating aspect. It allows light to pass through while maintaining its impressive insulating properties. This unique combination of features makes it a candidate for applications in optics and windows. Imagine having windows that not only insulate your home but also provide abundant natural light.
Applications of Silica Aerogel
Oil Spill Cleanup
One of the most notable applications of silica aerogel is in environmental remediation, particularly for oil spills. Silica aerogels can selectively absorb hydrophobic substances, such as oil, while repelling water. This property makes them highly efficient at cleaning up oil spills, mitigating environmental damage, and reducing the impact on aquatic ecosystems.
Construction and Energy Efficiency
In the realm of construction, silica aerogel is a game-changer. It can be incorporated into building materials to enhance their thermal insulation properties. Transparent aerogels are also being explored as window insulation materials, promising significant energy savings in buildings by reducing heating and cooling costs.
Space Exploration
Silica aerogels have played a pivotal role in space exploration. NASA has used them to capture cosmic dust particles during missions. Their exceptional thermal insulation properties have protected sensitive equipment in spacecraft, allowing them to endure the rigors of space travel. This makes silica aerogel an unsung hero of interstellar exploration.
Future Prospects
As we look to the future, silica aerogel holds immense promise in various domains:
Nanotechnology and Electronics
The nanotechnology and electronics industries are set to benefit from silica aerogel's unique properties. Its high surface area and porosity make it an excellent candidate for nanoscale applications, such as catalysis and energy storage. In electronics, silica aerogels could find use in advanced batteries and supercapacitors.
Medical and Biotechnology
Silica aerogels are finding their way into the medical and biotechnology sectors. Their biocompatibility and porous nature make them suitable for drug delivery, tissue engineering, and diagnostics. Researchers are exploring innovative ways to harness these properties for medical advancements.
Environmental Remediation
Beyond oil spill cleanup, silica aerogels have potential applications in addressing other environmental challenges. They could be used to remove pollutants from water sources or capture greenhouse gases, contributing to a cleaner and more sustainable future.
In conclusion, silica aerogel is a material that has captivated the world of chemical engineering with its extraordinary features, diverse applications, and exciting future prospects. As scientists and engineers continue to unlock its potential, we can expect to see silica aerogel making a significant impact across various industries, from space exploration to environmental conservation. The future looks promising, and silica aerogel is at the forefront of innovation.
Selected Further Reading
- Shi, H., Cui, J., Shen, H., & Wu, H. (2014). Preparation of Silica Aerogel and Its Adsorption Performance to Organic Molecule. Advances in Materials Science and Engineering, Volume 2014, 1–8.
- Sivri, S., Dilek, C., & Sezgi, N. A. (2019). Synthesis and Characterization of Aluminum Containing Silica Aerogel Catalysts for Degradation of PLA. International Journal of Chemical Reactor Engineering, 20180163, 1–10.
- Tamon, H., Kitamura, T., & Okazaki, M. (1998). Preparation of Silica Aerogel from TEOS, Journal of Colloid and Interference Science. 359(197), 353–359.
- Wang, L. J., Zhao, S. Y., & Yang, M. (2008). Structural characteristics and thermal conductivity of ambient pressure dried silica aerogels with one-step solvent exchange/surface modification. Materials Chemistry and Physics, 113(1), 485–490.
- Schüth, Ferdi & Schmidt, W. (2002). Microporous and Mesoporous Materials. Advanced Engineering Materials, 4, No.5, 269–279
- Pajonk, G. M. (2003). Some applications of silica aerogels. Colloid and Polymer Science, 281(7), 637–651.
Wednesday, 6 September 2023
Hydrogen Production: Natural Gas Reforming
This is a vital technology pathway for hydrogen production. Methane (CH4) that is contained in natural gas can be used to produce hydrogen through thermal processes like steam-methane reformation and partial oxidation.
a) Steam-Methane
Reforming
This
is the most common and cost-effective method for hydrogen production, and it
contributes to about 50% of the world's hydrogen production.
In
this production process, high-temperature steam (700°C–1,000°C) is used to
produce hydrogen from a methane source like natural gas. In this process
methane reacts with steam under 3–25 bar pressure (1 bar = 14.5 psi) in the
presence of a catalyst. This results in the production of hydrogen, carbon
monoxide, and a small amount of carbon dioxide. Heat is supplied to the steam
reforming process hence it is endothermic. Nickel is a common
catalyst used in this process. The catalyst being considered must have a high
surface area to volume ratio as there may be diffusion limitations at high
temperatures. The catalyst shape is also important in order to have a low
pressure drop, such shapes may be rings with holes and gear wheels.
In
the second step, the carbon monoxide and steam react in the presence of a
catalyst to produce carbon dioxide and more hydrogen. This is a process called water-gas
shift reaction. These two processes are represented in the following
equations:
In
the final step, carbon dioxide and other impurities are removed from the gas
stream to leave a pure hydrogen stream. This process is called pressure-swing
adsorption. Other fuels like ethanol, propane and gasoline can also
undergo steam reforming to produce hydrogen.
Although Steam-methane reforming is a mature technology, it suffers from noteworthy disadvantages such as mass and heat transfer issues and coke deposition during the reaction
a) Partial
Oxidation
In
this process methane and other hydrocarbons in natural gas react with an oxygen
amount that is less to the stoichiometric amount of oxygen needed, thus it doesn’t
completely oxidize into carbon dioxide and water. The limited amount of oxygen
for oxidation produces mainly carbon monoxide, hydrogen ad small amounts of
nitrogen, carbon dioxide and other compounds.
Then,
water-gas shift reaction is carried out to form carbon dioxide and more hydrogen.
In
this process, heat is given off hence it’s an exothermic process.
This
process is faster than steam reforming and requires a smaller reactor. However,
this process initially produces less hydrogen per unit of fed fuel than steam
reforming of the same amount of fed fuel.
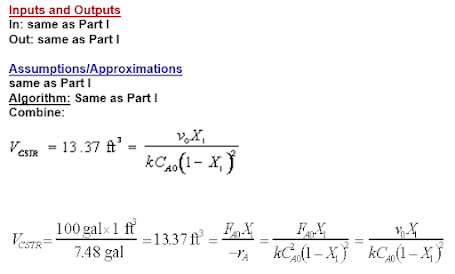
Maxwell Equation Solved Example
Example Show that: Solution This example uses the Maxwell Relations Table from the previous blog entry. You can read it here Lets combine ...

-
Fluid mechanics equations helps to predict the behavior of fluids in various flow situations. A fluid can be defined as a substance that de...
-
Let us determine the pressure at any point in a fluid a rest. For this, let us consider a wedge-shaped particle that is exposed on all sides...
-
1. Vapour Power Cycles Power cycle is a process where devices are used to continuously produce power. A typical vapor power cycle is the st...