This is a vital technology pathway for hydrogen production. Methane (CH4) that is contained in natural gas can be used to produce hydrogen through thermal processes like steam-methane reformation and partial oxidation.
a) Steam-Methane
Reforming
This
is the most common and cost-effective method for hydrogen production, and it
contributes to about 50% of the world's hydrogen production.
In
this production process, high-temperature steam (700°C–1,000°C) is used to
produce hydrogen from a methane source like natural gas. In this process
methane reacts with steam under 3–25 bar pressure (1 bar = 14.5 psi) in the
presence of a catalyst. This results in the production of hydrogen, carbon
monoxide, and a small amount of carbon dioxide. Heat is supplied to the steam
reforming process hence it is endothermic. Nickel is a common
catalyst used in this process. The catalyst being considered must have a high
surface area to volume ratio as there may be diffusion limitations at high
temperatures. The catalyst shape is also important in order to have a low
pressure drop, such shapes may be rings with holes and gear wheels.
In
the second step, the carbon monoxide and steam react in the presence of a
catalyst to produce carbon dioxide and more hydrogen. This is a process called water-gas
shift reaction. These two processes are represented in the following
equations:
In
the final step, carbon dioxide and other impurities are removed from the gas
stream to leave a pure hydrogen stream. This process is called pressure-swing
adsorption. Other fuels like ethanol, propane and gasoline can also
undergo steam reforming to produce hydrogen.
Although Steam-methane reforming is a mature technology, it suffers from noteworthy disadvantages such as mass and heat transfer issues and coke deposition during the reaction
a) Partial
Oxidation
In
this process methane and other hydrocarbons in natural gas react with an oxygen
amount that is less to the stoichiometric amount of oxygen needed, thus it doesn’t
completely oxidize into carbon dioxide and water. The limited amount of oxygen
for oxidation produces mainly carbon monoxide, hydrogen ad small amounts of
nitrogen, carbon dioxide and other compounds.
Then,
water-gas shift reaction is carried out to form carbon dioxide and more hydrogen.
In
this process, heat is given off hence it’s an exothermic process.
This
process is faster than steam reforming and requires a smaller reactor. However,
this process initially produces less hydrogen per unit of fed fuel than steam
reforming of the same amount of fed fuel.
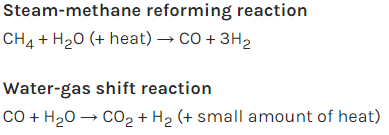





No comments:
Post a Comment