Mass transfer is the movement of particles or molecules from one point to another under the influence or a concentration gradient i.e., from an area of high concentration to an area of low concentration. This movement of particles occurs due to two mechanisms: Molecular diffusion and mass convention.
Molecular Diffusion
This refers to the movement of individual molecules
through a group of molecules without the aid of a bulk fluid flow like stirring.
Imagine a few molecules of a liquid A placed in a particular location within a larger
group of molecules of liquid B. As the molecules undergo movement through
Brownian Motion, the molecules of A will eventually be distributed into various
locations within liquid B as shown below:
The rate of transfer of molecules of A through
molecules of B from one location to another is through molecular diffusion.
This is mathematically described using Fick's Law. This is simplified into rate
of transfer of species A by molecular diffusion in the x-direction as shown in
the molecular diffusion equation below:
Where:
ṄA,x = diffusion transfer rate of species A
(moles transferred per unit time e.g., gmol/s) across area A in the x direction
between locations 1 and 2.
A = cross sectional area across which the diffusion
occurs (perpendicular to the x direction)
DAB = the binary diffusivity coefficient of
species A in species B with units of area per time (e.g., m2/s)
cA = concentration of species A (e.g.,
gmol/mol)
The figure below shows the molecular diffusion of a
molecule in the x direction through a cross sectional area, A.
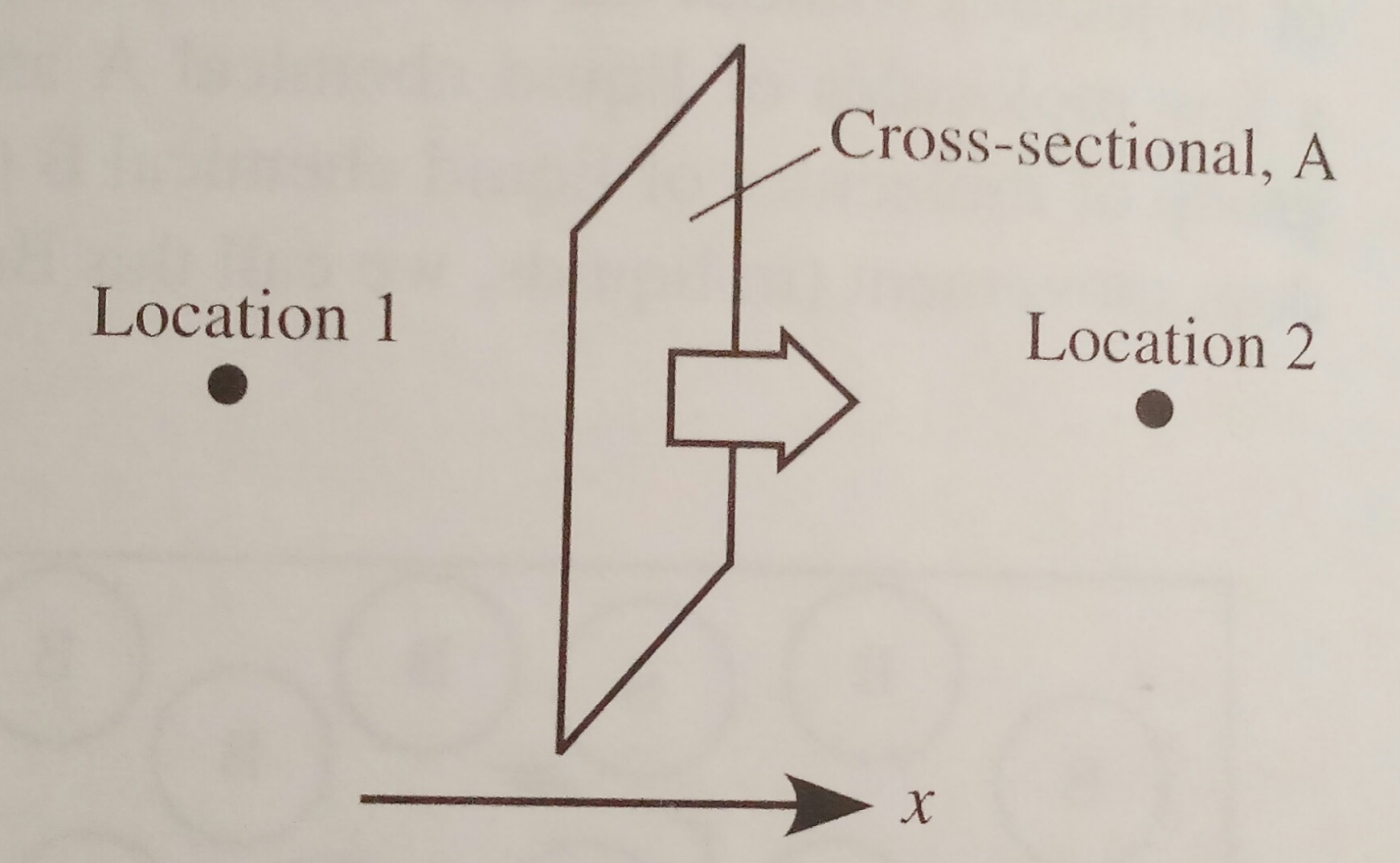
The transfer rate can be simplified in terms of a
driving force (ΔcA), which tends to produce the diffusion, and a
resistance (Δx/DABA), which tends to oppose the diffusion. The
binary diffusivity, DAB, describes the ease with which a molecule of
species A moves through the molecules of species B. Thus, when DAB
is large and transfer occurs rapidly, the resistance is small and when DAB
is small and transfer occurs slowly, the resistance is large. DAB is
not a constant and it varies with the physical conditions like temperature of
the system. The temperature affects the motion of the molecules. Thus, the
factors affecting DAB depend on the properties of the molecules of A
and B and are listed below:
- molecular size – this determines the distances and spaces between the molecules
- molecular shape – includes the presence of long chains that can tangle
- molecular charge – affects the attractive or repulsive forces between the molecules.
Mass Convection
This is the method by which velocities and flow help
the molecules of different types mix. In the figure below we can see a small
current of fluid moving in the direction represented by the dark arrow which
carries the molecules in its path to the new locations. Even though the
molecules undergo molecular diffusion, the convection dominates as a transfer
mechanism.
Mass convection distributes molecules faster than
molecular diffusion alone.
A frequent application of mass transfer in chemical
engineering processes is the transfer of materials across a phase boundary
(interface) where two phases (solid, liquid and/or gas) meet. In the design of
process that have mass transfer across phase boundaries, chemical engineers
include mass convection flow to increase that transfer. In mass transfer by
convection at phase boundaries, the net effect is the transport perpendicular
to the phase boundary as shown below, where the net transfer direction is indicated
by the large arrow. In this figure, the transfer mode of species A in Phase I
is not specified in order to focus on Phase II.
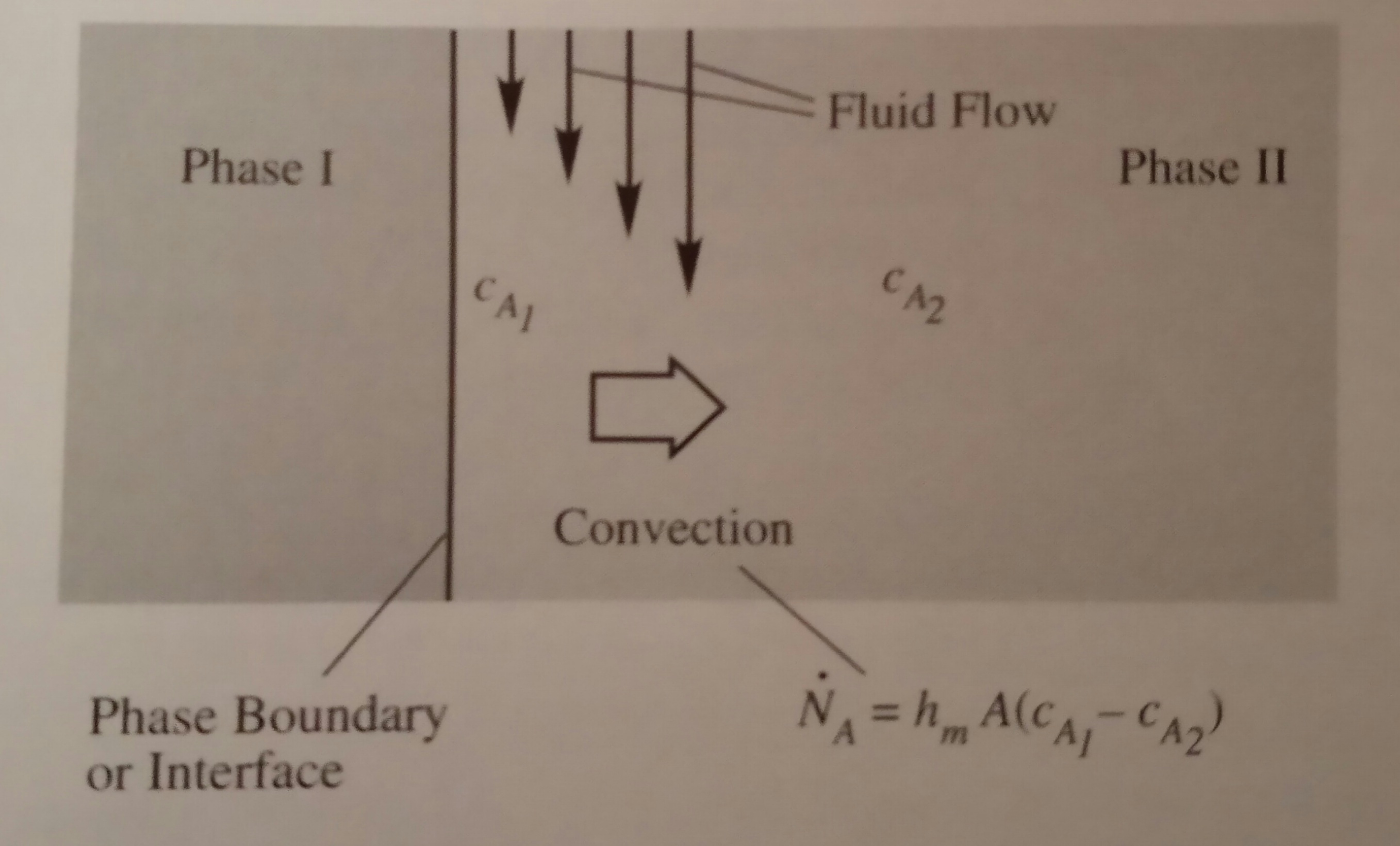
The following equation for mass convection
quantitatively describes the transport to/from phase boundaries via convection:
where:
cA1 = concentration of species A at the
starting location (1) of transfer (at the phase boundary)
cA2 = concentration of species A at the
ending location (2) of transfer (at the bulk of fluid)
ṄA = convection transfer rate of species A
through area A from locations 1 to 2
hm = mass transfer coefficient, which
accounts for the effects of diffusion and fluid motion (unit length per time)
A = cross sectional area through which the transfer
occurs
In the equation of mass convection shown above, the driving force is present in the concentration difference just as in the molecular diffusion equation. For mass convection, the resistance is 1/hmA. Thus, if hm is large, the resistance is small and transfer occurs rapidly and when hm is small, the resistance is large and transfer occurs slowly.









