For most substances, the thermodynamic properties are related to one another in a complex manner and cannot be expressed by simple equations. Thus, the properties are presented in tabulated form.
Some thermodynamic properties are easy to measure while others aren’t measured directly and need to be calculated using the relations between them and the measurable properties. The results of these measurements and calculations are then presented in tables. Some of the major properties are discussed below:
Enthalpy
In
an open system, Enthalpy (H) can be defined as the amount of energy transferred
across a system boundary by a moving flow. It is expressed as follows;
H
= U + PV
The
term PV represents the flow work and has the units of energy. Thus, H also has
the units of energy. Enthalpy is a state function since U, P and V are
all state functions, any combination of them must also be a state function.
Enthalpy is also an extensive property. Other expressions of enthalpy
are as follows;
Saturated
Liquid and Saturated Vapor
The
properties of saturated liquid and saturated vapor are usually presented in
tabulated form and presented in appendices of many Thermodynamics books. These
tables are usually either listed as either temperature and pressure tables. Thus,
it is convenient to use Saturated Water: Temperature Table when temperature is
given, and Saturated Water: Pressure Table when pressure is given.
The superscript L is used to denote the properties of a saturated liquid, and the superscript V to denote the properties of saturated vapor. The difference between the saturated vapor and saturated liquid states are designated by Δ, i.e.,
or;
The
quality of a saturated liquid is 0, the quality of a saturated vapor is 1.
It
is important to note that quality has no meaning in the subcooled (or,
compressed) liquid and superheated vapor regions.
In
the two-phase region, application of Gibbs phase rule gives, 2 + F = 1 + 2,
thus F = 1. Hence, if one of the independent intensive variables of a two-phase
mixture is known it is possible to specify the state of the system.
Superheated
Vapor
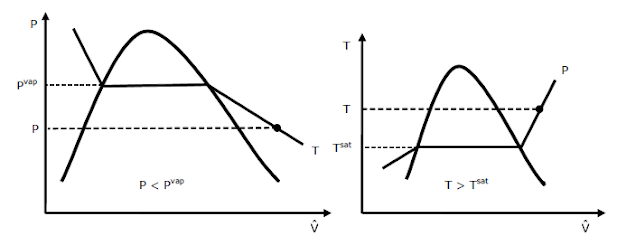
In
the region to the right of the saturated vapor line, a pure substance exists as
superheated vapor. In this region
P
< Pvap at a given temperature
T
> Tsat at a given pressure
This
is represented graphically as shown below:
The
degrees of superheat is defined as the temperature in excess of the saturation
temperature at a given pressure, i.e.,
Degrees
of Superheat = T - Tsat
For
a superheated vapor, application of Gibbs phase rule gives 1 + F = 1+2 ⇒ F = 2
Thus,
two independent intensive variables are required to specify the state of a
superheated vapor.
Subcooled
(Compressed) Liquid
In
the region to the left of the saturated liquid line, a pure substance exists as
subcooled (or, compressed) liquid. In this region
P
> Pvap at a given temperature (compressed liquid)
T
< Tsat at a given pressure (subcooled liquid)
This
is represented graphically as shown below:
Note:
Compressed liquid implies that the pressure is greater than the saturation
pressure for a given temperature. Conversely, subcooled liquid implies that the
temperature is lower than the saturation temperature for the given pressure.
As
an approximation, properties of compressed liquid are equal to that of a
saturated liquid at the given temperature. For a compressed liquid, application
of Gibbs phase rule gives 1 + F = 1+2 ⇒ F
= 2
Thus,
two independent intensive variables are required to specify the state of a
compressed (or subcooled) liquid.







No comments:
Post a Comment