Flux is defined as the amount of a quantity that is transported
per unit time across a unit area that is perpendicular to the direction of
transport. The molar flux of species i with units’ moles/m2.s is
represented as:
Where:
Ni-mol is the molar of species i
ui is the velocity of i with respect to a fixed
reference frame.
Similarly, the mass flux, Ni-mass, with units mass/m2.s
is represented as:
In some cases, it is convenient to interpret the total flux of
species i with respect to an arbitrary reference frame rather than a fixed set
of reference frame.
The molar flux of species i based on an arbitrary reference
velocity u0 is denoted by Ji-mol and is defined as:
Similarly mass flux of species i based on arbitrary reference
velocity u0 is denoted by Ji-mass which can be expressed
as:
In a system a frame of moving reference must be chosen, since
several molecular species move with different average velocities. The important
moving references are mass average, molar average and volume average
velocities.
Mass average velocity
This can be defined in terms of the mass concentration and the velocity
of species i based on a fixed axis. It is expressed as:
Molar average velocity
This can be expressed by the expression analogous to the mass
average velocity. It can be represented by replacing the mass concentration of
species i, ρi with the molar concentration of species i, Ci:
Volume average velocity
This is important for experimental analysis in a fixed system of
constant volume. The volume average velocity can be expressed by:
where vi is the partial molar volume of species i.
Relation Between Fluxes
The molar flux of species i described previously can be obtained
with respect to the molar average velocity as follows:
Substituting the molar flux of species i into the above equation
and rearranging it results in:
Substituting the definition of molar average velocity into the
above equation we get:
or:








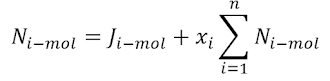




No comments:
Post a Comment